Very strong silicon-oxygen covalent bonds have to be broken throughout the structure before melting occurs. What are the Physical Properties of Silicon.
 Physical Properties Of Sio2 Xero Gel 39 40 41 Download Table
Physical Properties Of Sio2 Xero Gel 39 40 41 Download Table
Physical mechanical thermal and electrical properties of quartz and fused silica.
Physical properties of silicon dioxide. It is also called Silica or Kalii bromidum or Silicic oxide or silicic acid. Physical Properties of SiO2 Silicon dioxide is transparent to gray crystalline odorless or an amorphous solid. Its structure is similar to diamond see table below.
It is insoluble in water and acid. The Physical Properties of Silicon IV Oxide are as Follows. They have melting and boiling points as 1713o C and 2950o C respectively.
The atomic number of silicon is 14 and its relative atomic mass is 28085 u. Silica is the most abundant mineral in the Earths crust. The noncrystalline phase likes to develop towards the thermodynamic equilibrium structure by the process is known as the devitrification is explained.
With hydrogen silicon forms a series of hydrides the silanes. The Physical properties of Silicon are the characteristics that can be observed without changing the substance into another substance. Silicon dioxide SiO 2 also exists as a covalent network and is known as quartz or silica.
It is hard and has a high melting point but contains silicon and. Its melting and boiling point are 1600 oC and 2230 oC respectively. Nano-silica particles are divided into P-type and S-type according to their structure.
At red heat silicon is attacked by water vapour or by oxygen forming a surface layer of silicon dioxide. Silicon dioxide nanoparticles also known as silica nanoparticles or nano-silica are the basis for a great deal of biomedical research due to their stability low toxicity and ability to be functionalized with a range of molecules and polymers. It is obtained as a transparent to grey in its crystalline or amorphous powdered form.
Diatomaceous earth flux-calcined filter aid flux calcined treated with sodium carbonate. Silica or silicon dioxide which is found in sand has a similar structure to diamond so its properties are similar to diamond. Has a high melting point - varying depending on what the particular structure is remember that the structure given is only one of three possible structures but around 1700C.
Physical Properties of Covalent Network Solids The elements carbon silicon and boron form covalent networks instead of covalent molecules. Silicon dioxide 99 05-10 mum approx. Silicon Dioxide does not conduct electricity since there arent any delocalized electrons with all the electrons are held tightly between the atoms and are not free to moveSilicon Dioxide is insoluble in water and organic solvents.
Silica is most commonly found in nature as sand or quartz as well as in the cell walls of diatoms. Crystalline silicon has the same structure as diamond. Silicon dioxide is a giant covalent structure.
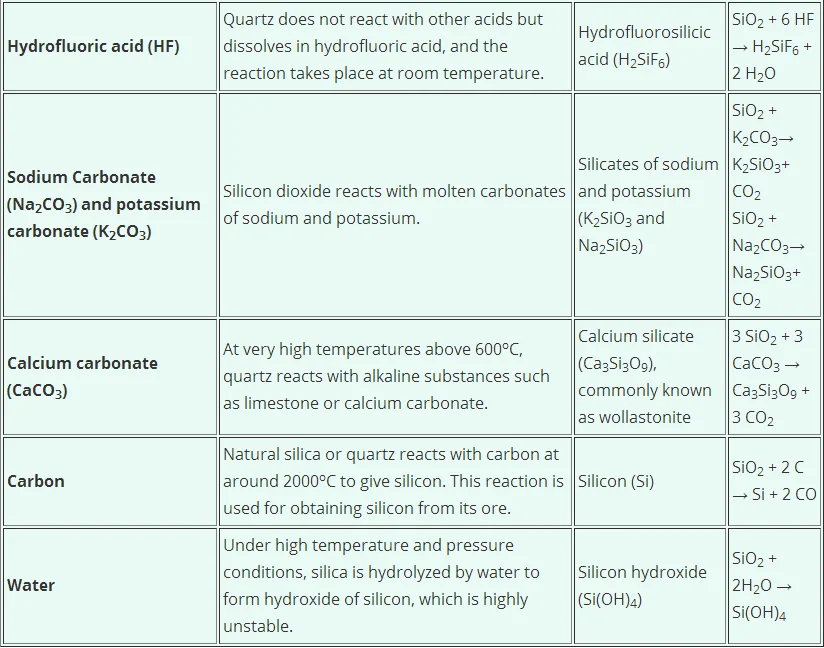
However it is soluble in alkalies and hydrofluoric acid. Its density is 265 g mL-1. The physical properties of silicon dioxide.
Silica is a group IV metal oxide which has good abrasion resistance electrical insulation and high thermal stability. The chemical compound silicon dioxide also known as silica from the Latin silex is an oxide of silicon with a chemical formula of SiO2 and has been known for its hardness since antiquity. Structure and properties of Silicon Dioxides are explained in detail.
It is insoluble in all acids with the exception of hydrogen fluoride HF. The easiest one to remember and draw is based on the diamond structure. There are three different crystal forms of silicon dioxide.
80 between 1-5 mum Silicon dioxide amorphous cyclic azasilanehexamethyldisilazane treated. The density is about 2648 gcm3. The Physical Properties of Silicon are as follows.
The thermally grown silicon dioxide on silicon is amorphous. The table given below enlists the values for some of the physical properties of silicon dioxide both crystalline and amorphous. This oxide is a macromolecular compound that has the oxygen and silicon atoms linked together covalently in what is known as tetrahedral basic units.
Physical properties are usually those that can be observed using our senses such as color luster freezing point boiling point melting point density hardness and odor. SiO 2 is an oxide of silicon with a chemical name Silicon Dioxide. Physical Properties of Silicon One silicons allotrope is in the form of needle-like shiny greyish-black crystals or flat plates while the other one has no crystal structure and it exists usually as a brown powder.
Silicon IV oxide exists as colorless crystalline solid in its pure state. Silicon dioxide is insoluble in water and in all organic solvents. It is widely found in nature as quartz.
Silicon dioxide is a transparent to gray odorless crystalline or amorphous solid. When silicon and carbon are combined at electric furnace temperatures 20002600 C 36004700 F they form silicon carbide carborundum SiC which is an important abrasive. Silicon dioxide fused granular 4-20 mesh 999 trace metals basis.