Increasing the temperature of the solvent was expected to increase the pressure of the vapor above the solvent. Vapor Pressure and Heat of Vaporization Objective.
Hvap is the change in heat vaporization R is the universal gas constant which is 8.
Vapor pressure lab report. Diploma in Chemical Semester. To achieve this a metal container was filled with water and was heated. This phenomenon is shown in many physical chemistry textbooks in plots of PV isotherms at temperatures below the critical temperature.
Vapor pressure of Water lab Report. FACULTY OF CHEMICAL ENGINEERING Universiti Teknologi MARA Cawangan Terengganu Kampus Bukit Besi Bukit Besi Dungun TERENGGANU TECHNICALEXECUTIVE REPORT. Please sign in or register to post comments.
Vapor Pressure and Heat Evaporation Lab Report Vapor Pressure and Heat of Vaporization Introduction. And calculate the heat of vaporization of the liquid. Wear safety goggles and lab apron at all times in lab.
CHEMICAL ENGINEERING Lab No. When a liquid is placed into a confined space some of the liquids will evaporate. Background As a chemical engineer you need strong intuition about how pressure is created in systems that are a pure component vs.
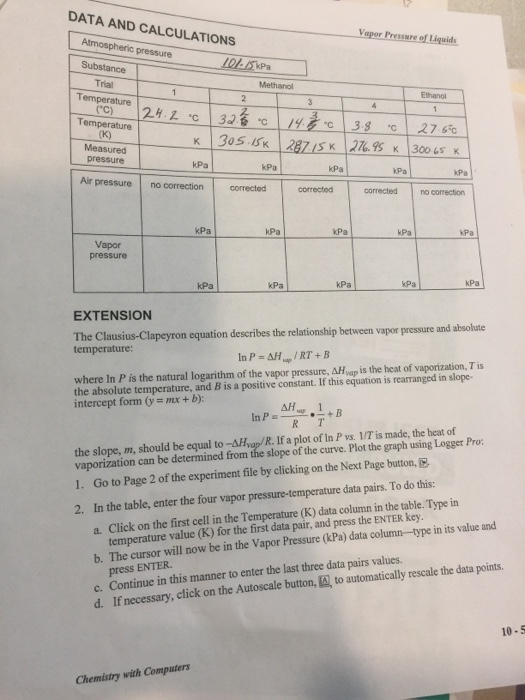
Determine the relationship between pressure and temperature of the volatile liquid. Vapor Pressure of Liquids - 1 - Experiment 6. When the natural logarithm of vapor pressure is plotted against the.
Here y ln P vapor unitless and x 1T in units of K-1. EQUATION 14-1 has the form of the equation for a straight line y mx b. Evaporation is the process of a liquid becoming vaporized.
Vapor Pressure of a Pure Liquid. The vapor pressure of a pure liquid is an intensive property of a compound. Principles of Chemistry CHEM 1111 Uploaded by.
Vapor is the vapor pressure R is the gas constant T is the temperature in Kelvins and C is a constant. In this exercise you will determine the enthalpy of vaporization D vap H of water and the effect of a non-electrolyte solute sucrose has on waters vapor pressure and D vap H. Name Matrix No.
31 JmolK T is the absolute or Kelvin temperature and C is the constant that is not related to heat capacity. 1212017 Participant Course. Vapor Pressure of Liquids Background Liquids contain molecules that have different kinetic energies due to different velocities.
2 Use a hot plate to heat 200 mL of water in. Development PSYC 163. Amanda Nienow adapted from Shoemaker et al.
Some of the faster liquid molecules have enough kinetic energy to vaporize. In this investigation we will examine the relationship between vapor pressure and temperature by considering the properties of benzene water and n-heptane at various temperatures and within a vacuum apparatus. A single component two phase system through measuring the vapor pressure as a function of temperature.
For the questions concerning the plot have answers. At the end of the hour after cleaning up get the TA to initial the end of your report. This Experiment was conducted to learn the relationship between Pressure and Temperature in a confined container.
615 560 550 410. Thermo lab report 1. As expected the vapor pressure increased as temperature increased.
Lab Report 5 Heat of dissociation of N2O4 Lab Report 4 Surface tension of solution Bomb Calorimeter Post Lab. Stir the water with the temperature probe and monitor the pressure and temperature readings. 01 Saturation Vapor Pressure Measurement Topic.
Vapor Pressure of Liquids Solutions Revised 121314 3 1. Thus the equilibrium between a pure liquid and its vapor shown in the equation. The boiling point corresponds to the temperature at which the vapor pressure.
This was clearly shown by the graphs of vapor pressure. Where 1nP is the natural logarithm of the vapor pressure. Measure the pressure inside a sealed vessel containing a volatile liquid over a range of temperatures.
Lab Report Continued 7 Calculations. Vapor pressure or saturation pressure is a temperature-dependent property of a. Vapor Pressure of a Pure Liquid.
The twelfth step is to add a small amount of hot water to warm the water bath by only a few degrees. EH1102A 6A Engineering No. HVAC R 1TC.
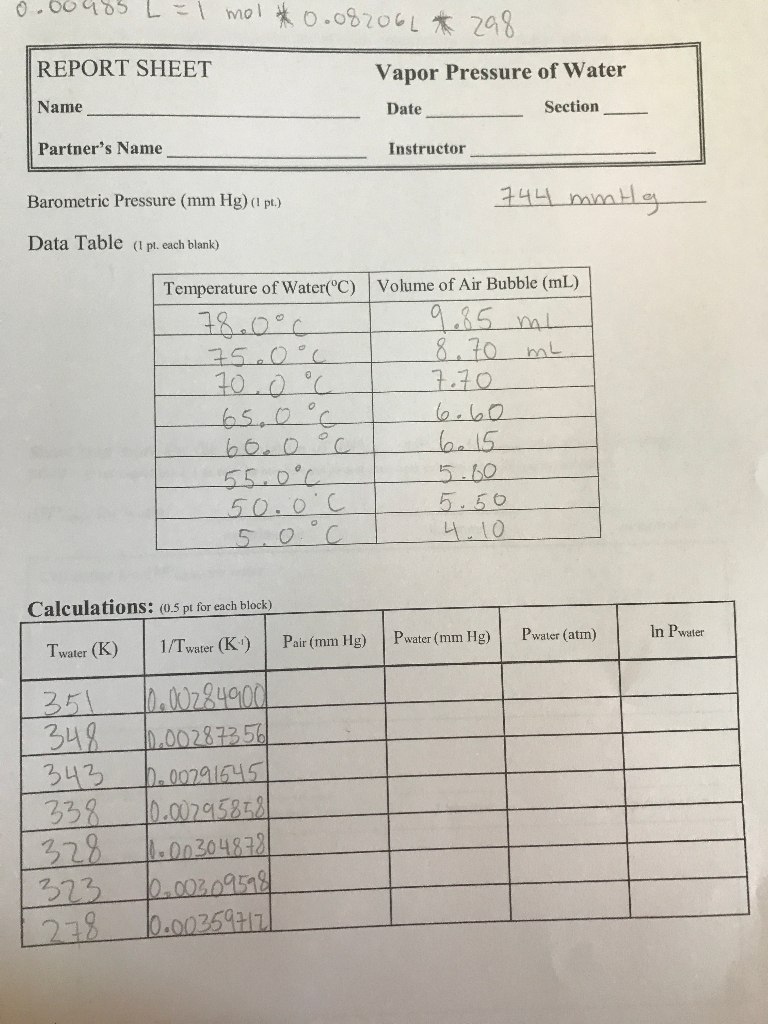
Vapor Pressure of Liquids Formal Report Abstract This lab explores the relationship between vapor pressure and temperature the vapor pressure and temperature of Methanol and Ethanol at the same temperature and the enthalpy of vaporization for Methanol and Ethanol. That is the vapor pressure of a liquid is independent of the amounts of the two phases as long as both phases are present. I TUJL-1 mol 0082066 298 REPORT SHEET Name Vapor Pressure of Water Date Section Partners Name Instructor Barometric Pressure mm Hg 1 pt 744 mmHg Data Table pl.
1 Obtain and wear goggles and gloves. Increase in temperature causes an increase in air pressure when the volume is maintained constant Benn. You are responsible for performing a literature search to find out more information on this system and why.
BC Chemistry 162 Laboratory Manual Experiment 6. Cach blank Temperature of WaterC 780C 750c 700C 6506 600C 550C 500C 50 Volume of Air Bubble mL 9 85 mL L 870 mL L 770. Thermo lab report 1.
1-4 from the presentation Note. Lab report as activities are completed. Place the Temperature Probe in the water bath and monitor the pressure and temperature readings.
Vapor Pressure that is independent of the quantity of the liquid or vapor. Calculate air and vapor pressure as you collect each pressure temperature data pair see Calculations 1 many students get erroneous results for this experiment so calculating as you go will enable you to.