1 answer below Define a pure compound refrigerant and give two examples. CFC are pure compound refrigerants.
 Chapter 3 Pure Substance Ppt Download
Chapter 3 Pure Substance Ppt Download
The 2013 Cadillac XTS was the first USmade vehicle to use the new refrigerant.

What is a pure compound refrigerant. More_vert Define a pure compound refrigerant and give two examples. Figure5 shows a pure compound CFC- 12 and a near-azeotropic HCFC-based. CFC are pure compound refrigerants.
A refrigerant may be either a pure compound or a mixture blend of two or more refrigerants. Define pure compound refrigerant. Define a pure compound refrigerant and give two examples.
Define pure compound refrigerant. A pure compound refrigerant maintains its form in pressure and temperature regardless of any changes of phase applied to it. A mixture can behave either as a pure refrigerant azeotropic mixtures or differently non-azeotropic or zeotropic mixtures.
The R-134a molecule is ethane-based and consists of carbon C fluorine F and hydrogen H. Similarly it is asked is 134a refrigerant a blend. A refrigerant may be either a pure compound or a mixture blend of two or more refrigerants.
With a GWP of 4 instead of 1430 for R-134a R-1234yf is more environmentally friendly. Refrigeration and Air Conditioning. A refrigerant is a substance or mixture usually a fluid used in a heat pump and refrigeration cycle.
It is considered a pure compound and has only one molecule. A refrigerant that does not change its pressuretemperature relationship when changing phases. As in the example above a molecule of pure water always contains two hydrogen atoms and one oxygen atom H 2 O.
What are two examples of pure compound. However research proved that most blends were near-azeotropic enough for fractionation to be managed Figure5. A mixture is made up of two or more pure compounds with distinct chemical properties.
It is considered a pure compound and has only one molecule. Mechanical Engineering Refrigeration and Air Conditioning Technology MindTap Course List Define a pure compound refrigerant and give two examples. Examples of mixtures are R502 R404A and R407C.
Fluorocarbons especially chlorofluorocarbons became commonplace in the 20th century but they are being phased out because of their ozone depletion effects. Examples of pure refrigerants are R12 R22 and R134a. Oct 23 2017 0653 AM.
However unlike R134a which is a pure compound R407C has a glide of 7 K making it barely usable in small residential household equipment. A mixture can behave either as a pure refrigerant azeotropic mixtures or differently non-azeotropic or zeotropic mixtures. Examples of mixtures are R502 R404A and R407C.
An azeotropic refrigerant is a mixture of two or more components that boil at the same temperature. Examples of pure refrigerants are R12 R22 and R134a. R407C is like R134a thermodynamically similar to R22 and works as a drop in refrigerant.
Define net refrigeration effect as it applies to the refrigeration cycle. A mixture can behave either as a pure refrigerant azeotropic mixtures or differently non-azeotropic or zeotropic mixtures. Therefore a pure compound simply indicates that a substance includes two or more elements in a specific ratio which never varies.
Examples of mixtures are R502 R404A and R407C. R-134a is not a refrigerant blend. Other common refrigerants used in various applications are ammonia sulfur dioxide and non-halogen.
Changes from gas to liquid and then back to gas in the refrigeration cycle. R-134a is not a refrigerant blend. While many other molecules contain both hydrogen and oxygen only water contains those elements in that exact 21 ratio.
In most cycles it undergoes phase transitions from a liquid to a gas and back again. A mixture can behave either as a pure refrigerant azeotropic mixtures or differently non-azeotropic or zeotropic mixtures. A refrigerant may be either a pure compound or a mixture blend of two or more refrigerants.
Define a pure compound refrigerant and give two examples. Refrigerant blend fractionation may also result in faster capacitylosses than single component pure compounds like CFC- 12502 or HCFC-22. The components in this type of refrigerant will evaporate and condense together as one.
Many working fluids have been used for such purposes. It is an azotroptic refrigerant blend that has negligible temperature glide and behaves like a pure compound. A refrigerant that does not change its pressuretemperature relationship when changing phases.
Not all refrigerants are pure compounds such as R22 and R134a and not all refrigerants are created equal. A refrigerant is a chemical compound that is used as the heat carrier. Examples of pure refrigerants are R12 R22 and R134a.
What is a pure refrigerant. A pure compound is made up entirely of one substance that has a single chemical property. 25Define a pure compound refrigerant and give two examples.
A pure compound refrigerant.
Is A Mixture Of Air And Liquid Air A Pure Substance Quora
 5 7 Azeotropic Zeotropic Refrigerants Swep
5 7 Azeotropic Zeotropic Refrigerants Swep
 Solved Choose The Correct Answer From The Following Each Chegg Com
Solved Choose The Correct Answer From The Following Each Chegg Com
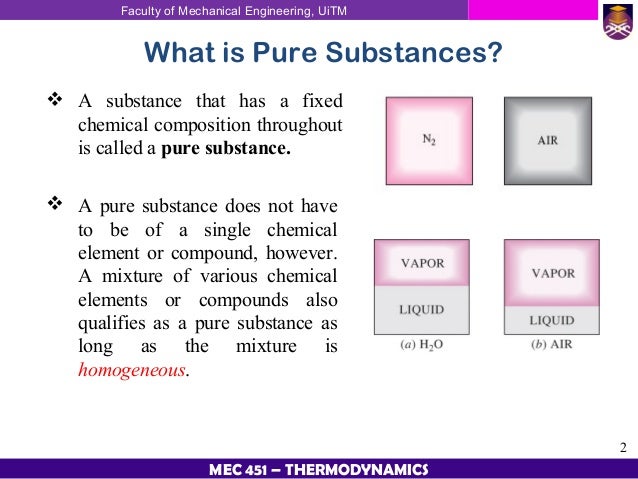 Thermodynamic Chapter 2 Properties Of Pure Substances
Thermodynamic Chapter 2 Properties Of Pure Substances
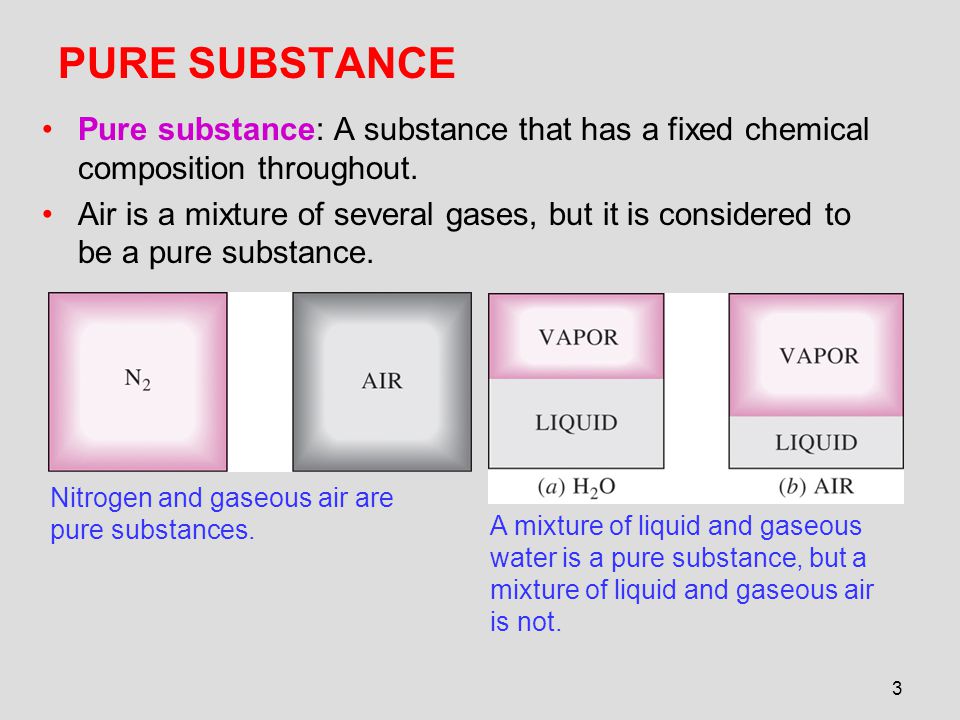 Chapter 3 Properties Of Pure Substances Ppt Video Online Download
Chapter 3 Properties Of Pure Substances Ppt Video Online Download
Properties Of Pure Substance Sounak Bhattacharjee
 Criteria For Refrigerant Selection
Criteria For Refrigerant Selection
 Inorganic Compounds Refrigerant Blog
Inorganic Compounds Refrigerant Blog
 Chapter 2a Pure Substances Phase Change Properties Updated 9 20 09
Chapter 2a Pure Substances Phase Change Properties Updated 9 20 09
Properties Of Pure Substance Sounak Bhattacharjee
 5 7 Azeotropic Zeotropic Refrigerants Swep
5 7 Azeotropic Zeotropic Refrigerants Swep
 Ch 2 Properties Of Pure Substances Objectives Introduce The Concept Of A Pure Substance Introduce The Concept Of A Pure Substance Discuss The Physics Ppt Download
Ch 2 Properties Of Pure Substances Objectives Introduce The Concept Of A Pure Substance Introduce The Concept Of A Pure Substance Discuss The Physics Ppt Download
ads


